X Chromosome Inactivation: Key to Treating Genetic Diseases
X chromosome inactivation is a fascinating biological process critical to understanding genetic disorders, particularly in females who possess two X chromosomes. This mechanism ensures that one of the X chromosomes is silenced to prevent an overexpression of genes located on it, which can lead to severe health complications. Recent advancements in chromosomal research have shed light on how this intricate silencing process occurs, providing hope for developing therapeutic treatments for conditions like Fragile X Syndrome and Rett Syndrome. As researchers delve deeper, the potentials of gene therapy emerge, promising innovative ways to tackle diseases rooted in X chromosome mutations. With thousands affected by such genetic disorders, the quest to harness the insights from X chromosome inactivation may hold the key to transformative medical therapies.
Exploring the concept of X chromosome inactivation reveals a critical mechanism in the realm of genetics and its implications for female health. This process, which involves the silencing of one X chromosome, plays a pivotal role in managing gene expression and maintaining cellular balance. By understanding how this silencing mechanism operates, researchers are opening doors to potential breakthroughs in addressing various genetic conditions, including well-known disorders like Fragile X and Rett syndrome. The ongoing investigations into chromosomal behavior not only contribute to our knowledge of gene regulation but also lay the groundwork for gene therapy innovations. Ultimately, these efforts may culminate in successful interventions for those impacted by chromosomal anomalies.
Understanding X Chromosome Inactivation: A Breakthrough in Genetic Research
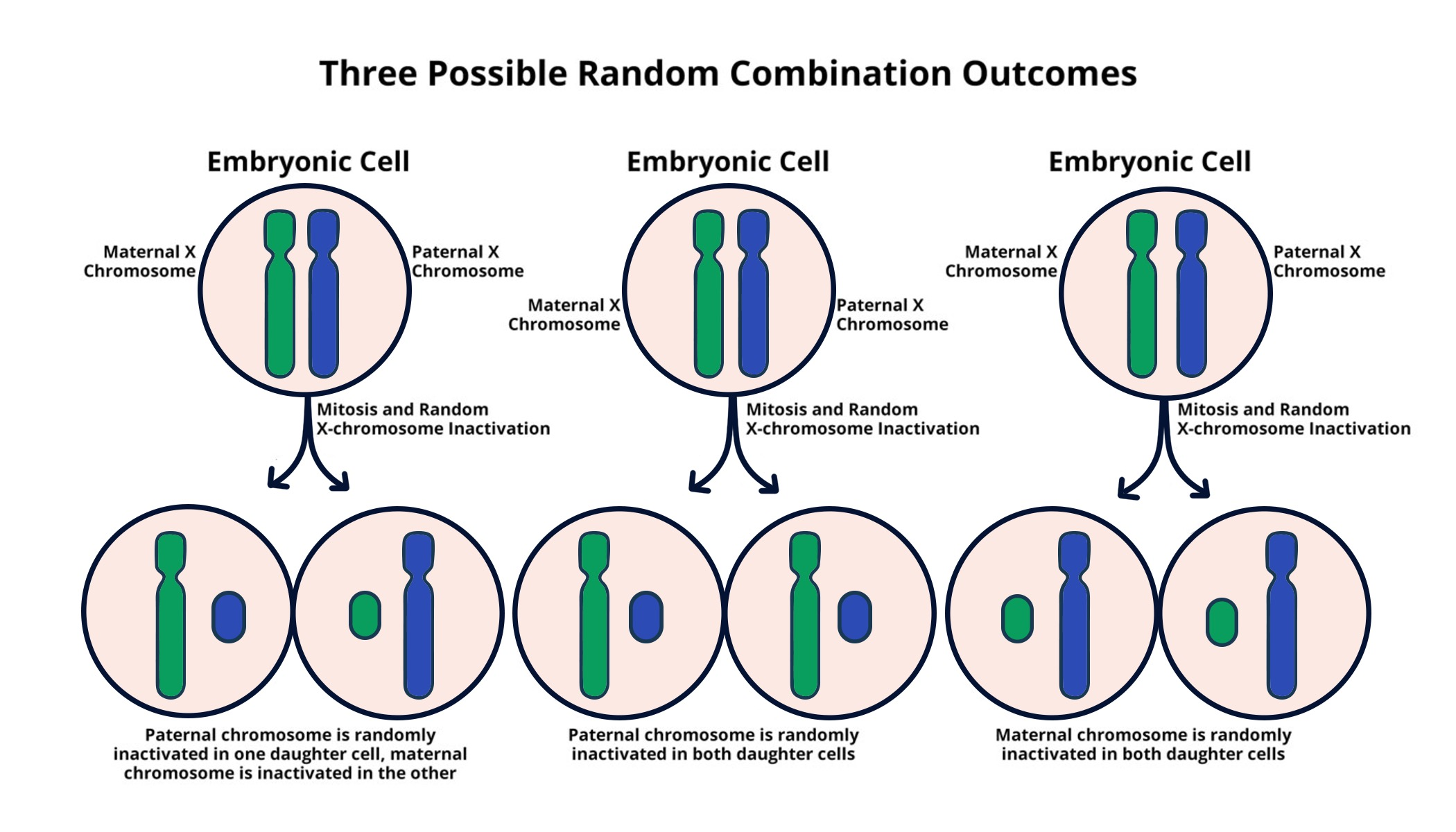
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females. In females, where two X chromosomes are present, one X chromosome becomes inactivated to prevent an overload of gene expression. This intriguing mechanism has been a focal point for researchers aiming to uncover the intricacies of genetic regulation. Jeannie T. Lee’s lab has made significant strides in this area, providing insights that could have far-reaching implications for treating chromosomal disorders like Fragile X syndrome and Rett syndrome. By unraveling how XCI occurs, researchers are one step closer to developing effective gene therapy techniques that could alleviate the burden of these genetic disorders.
Moreover, the complexity of XCI is heightened by the unique properties of the chromatin surrounding the X chromosome. The gelatinous substance that coats chromosomes—often likened to ‘Jell-O’—is critical for maintaining structural integrity while allowing for the nuanced cellular processes that govern gene expression. Understanding these interactions not only sheds light on foundational genetic mechanisms but also opens pathways for innovative treatments that could potentially reactivate silenced genes in affected individuals.
The exploration of X chromosome inactivation is particularly significant in the context of genetic disorders that affect both males and females differently. Conditions like Fragile X syndrome, which stems from mutations in the FMR1 gene on the X chromosome, highlight the urgent need for effective therapeutic strategies. By gaining deeper insights into how XCI operates, researchers can develop targeted gene therapies that focus on silencing the detrimental effects of mutated genes while preserving the function of their healthy counterparts. This dual focus on understanding both the molecular and therapeutic aspects of XCI could revolutionize approaches to treating a range of chromosomal disorders.
Gene Therapy Advances: Transforming Treatment for Genetic Disorders
Gene therapy has emerged as a beacon of hope for those suffering from genetic disorders, particularly X-linked conditions like Fragile X syndrome and Rett syndrome. By leveraging the body’s own genetic machinery, researchers aim to correct or compensate for faulty genes through innovative treatment methodologies. The findings from Jeannie T. Lee’s research into X chromosome inactivation and the potential for unsilencing mutated genes represent a significant leap forward in the field of genetic medicine. The prospect of freeing inactivated X chromosomes not only holds promise for individuals with these genetic conditions but also marks a critical advancement in chromosomal research.
As clinical trials for these therapies begin to take shape, the implications for patients and their families are profound. Successful gene therapy could lead to a newfound quality of life for individuals affected by intellectual disabilities and neurodevelopmental disorders. The overarching goal is to develop therapies that are not only effective but also safe, minimizing potential side effects while maximizing therapeutic benefits. As the scientific community continues to navigate the complexities of gene therapy, the hope is that techniques derived from the study of XCI will pave the way for new treatments, making a tangible difference in the lives of those impacted by genetic disorders.
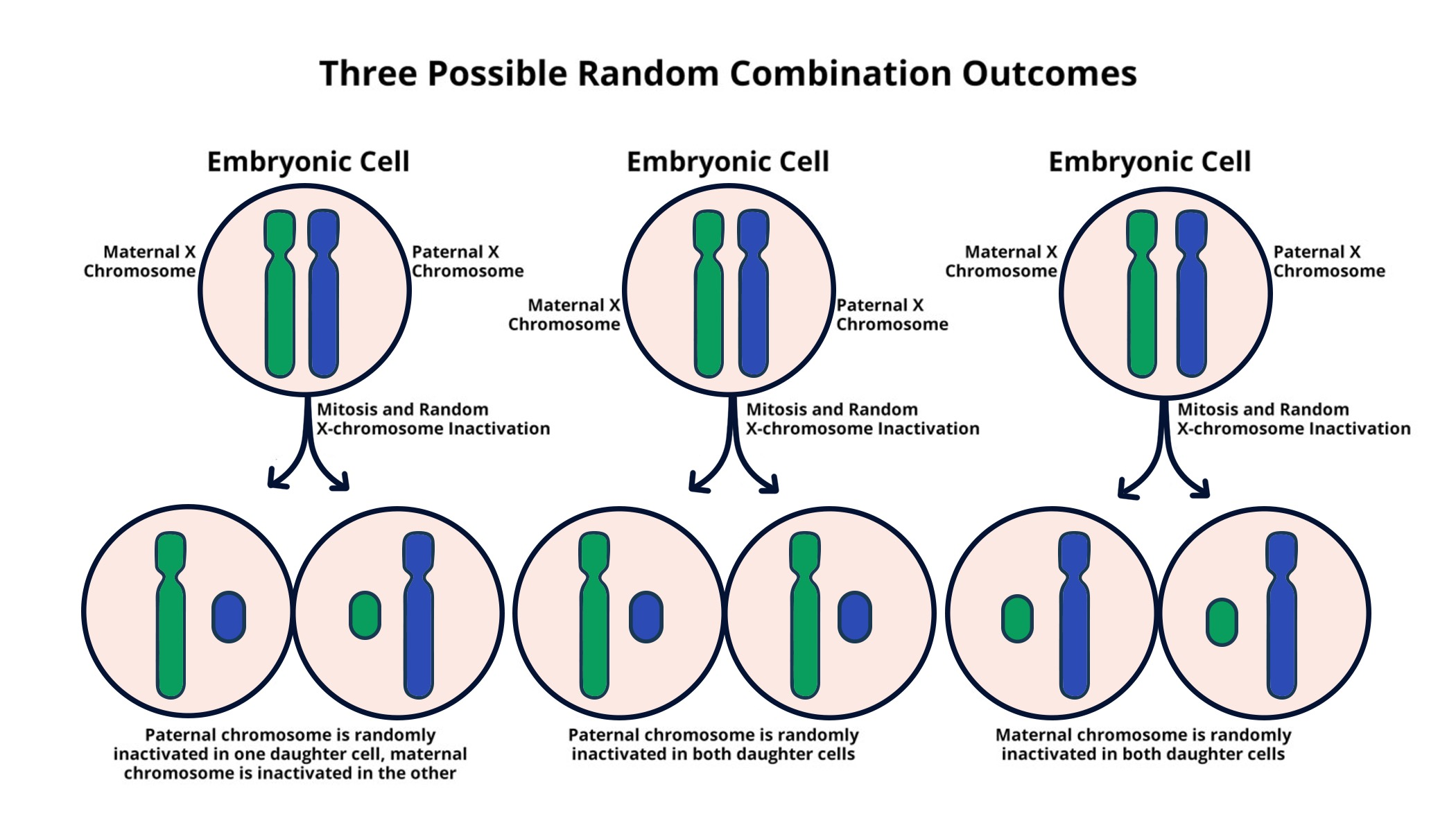
The Role of Xist RNA in X Chromosome Inactivation
At the heart of the X chromosome inactivation process lies the Xist RNA molecule, which plays a pivotal role in silencing the second X chromosome in females. Upon its transcription, Xist coats the X chromosome, instigating a series of structural changes that ultimately leads to gene inactivation. This fascinating molecular dance highlights the sophistication of genetic regulation and the intricacies involved in cellular decision-making. Studies led by researchers like Jeannie T. Lee have elucidated the mechanisms by which Xist interacts with the surrounding chromatin, often described as ‘chromosomal Jell-O’, making this research central to understanding genetic disorders.
Xist’s unique properties and functions extend beyond mere inactivation, as it facilitates the recruitment of other molecules that contribute to the gene silencing process. This capability not only underscores the importance of Xist in maintaining genomic stability but also suggests potential therapeutic targets for overcoming the challenges posed by genetic disorders. By understanding how Xist functions, scientists can explore strategies to manipulate this process, offering hope for developing gene therapies that can reactivate beneficial genes silenced due to mutations, particularly in the context of conditions such as Fragile X syndrome and Rett syndrome.
Chromosomal Research Leading to Innovative Therapies
Advancements in chromosomal research, particularly concerning the X chromosome, have significant implications for the development of novel therapeutic interventions. The understanding of X chromosome inactivation has catalyzed new approaches in gene therapy, enabling scientists to target specific genetic defects associated with disorders like Fragile X syndrome. As researchers like Jeannie T. Lee continue to investigate the mechanisms behind gene silencing and reactivation, the potential to design tailored therapies grows, aimed at restoring function to mutated genes without affecting their healthy counterparts.
Moreover, the interdisciplinary nature of chromosomal research encourages collaboration between geneticists, biochemists, and medical professionals. This unified approach paves the way for comprehensive studies that tackle both the basic science of XCI and its therapeutic applications. As ongoing research yields further insights, the prospect of translating these findings into clinical practice becomes increasingly realistic, promising innovative solutions for patients dealing with the challenges of genetic disorders. With continued investment and focus in this field, the hope remains that groundbreaking therapies will one day become available to those most in need.
Fragile X Syndrome: Challenges and Future Directions
Fragile X syndrome (FXS) serves as a prime example of the complexities involved in genetic disorders linked to the X chromosome. Caused by a mutation in the FMR1 gene, FXS is characterized by intellectual disability and a range of behavioral issues. Despite its recognition, the path to effective treatments has been fraught with challenges, many of which stem from the intricate nature of gene regulation and expression associated with XCI. Ongoing research seeks to not only understand the underlying mechanisms of FXS but also to innovate therapeutic strategies that leverage these insights.
As researchers delve deeper into the biology of Fragile X syndrome, they are also faced with the challenge of translating their findings into viable treatment options. Novel gene therapies are being explored that aim to revive silenced genes and alleviate symptoms associated with FXS. Moreover, the potential to develop targeted approaches that account for variations in gene expression across individuals is gaining traction in the scientific community. Continued exploration into the nuances of FXS and its relationship with X chromosome inactivation may ultimately lead to breakthroughs that transform the landscape of treatment for this genetic disorder.
Rett Syndrome: Understanding Its Genetic Basis
Rett syndrome is another neurodevelopmental disorder predominantly affecting females, characterized by a regression in speech and motor skills. At the genetic level, Rett syndrome is primarily caused by mutations in the MECP2 gene located on the X chromosome. As with Fragile X syndrome, understanding how X chromosome inactivation impacts the expression of the MECP2 gene is critical for developing effective therapies. Research efforts are focused on unraveling the connections between gene mutations and their phenotypic presentations, particularly concerning the dichotomy between affected females and asymptomatic males who carry the same mutations.
The implications of X chromosome inactivation in Rett syndrome are profound, as therapeutic strategies aimed at adjusting the expression of MECP2 could yield significant advancements in treatment options. Promising avenues include gene therapy approaches that restore normal function of the MECP2 gene by manipulating XCI dynamics. As the field evolves, continuous investigation into the relationships between genetic factors and clinical outcomes will be essential for advancing care for individuals with Rett syndrome and improving quality of life.
Innovations in Chromosomal Therapies: Potential Cures for Genetic Disorders
The rapid advancements in chromosomal therapies hold immense potential for curing genetic disorders, particularly those influenced by the X chromosome. With researchers like Jeannie T. Lee leading the charge, innovative approaches targeting X chromosome inactivation are being developed to address conditions such as Fragile X syndrome and Rett syndrome. These strategies range from using small molecules to interact with the chromatin structure to gene editing techniques aimed at repairing mutations within X-linked genes.
Clinical trials based on these pioneering research initiatives are poised to transform the management of genetic disorders. By focusing on unsilencing harmful mutations while preserving the function of healthy genes, scientists aim to create targeted treatments that can alleviate symptoms and improve overall patient outcomes. The collaborative nature of contemporary chromosomal research further accelerates this progress, uniting experts across disciplines to explore the therapeutic possibilities of understanding and manipulating X chromosome dynamics.
Future Implications of X Chromosome Research in Genetics
The future of genetics is closely intertwined with the continued research of the X chromosome and the mechanisms of X chromosome inactivation. As breakthroughs are made in understanding how genes are silenced and reactivated, the potential for novel gene therapies expands, ushering in a new era of treatment options for genetic disorders. This exploration not only impacts conditions like Fragile X syndrome and Rett syndrome but also extends to other genetic afflictions that share similar chromosomal dynamics, suggesting a broader application of therapeutic strategies arising from this research.
Furthermore, as genomic technologies advance, the ability to investigate complex genetic interactions and their phenotypic consequences will significantly improve. The integration of advanced computational models and big data analytics will enhance our understanding of how XCI affects gene expression patterns across different populations. This holistic approach to chromosomal research will be critical for tailoring effective therapies and ensuring that emerging treatments are both safe and efficacious for diverse patient groups.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic disorders?
X chromosome inactivation (XCI) is a biological process where one of the two X chromosomes in females is inactivated to balance gene dosage between males (who have one X chromosome) and females. This mechanism is crucial in understanding genetic disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome, as it affects how genes are expressed and can influence the severity of these conditions.
How might X chromosome inactivation be linked to gene therapy for Fragile X Syndrome?
Research into X chromosome inactivation has revealed that Xist RNA molecules play a pivotal role in silencing one of the X chromosomes. In gene therapy for Fragile X Syndrome, scientists aim to unsilence the inactivated chromosome to restore functionality to healthy genes that may be mutated, leading to potential treatments that could alleviate symptoms associated with this disorder.
What role does Xist play in the process of X chromosome inactivation?
Xist is a gene on the X chromosome that produces an RNA molecule crucial for X chromosome inactivation. It interacts with the chromosomal environment, changing the properties of the surrounding structure, referred to as ‘Jell-O,’ to ensure the inactivation process is effective. This mechanism is fundamental in prospects of developing therapies aimed at restoring gene function in conditions like Rett Syndrome.
Can understanding X chromosome inactivation lead to breakthroughs in treating genetic disorders?
Yes, insights into X chromosome inactivation can lead to breakthroughs in treating genetic disorders, especially those related to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. By focusing on how inactivation occurs, researchers like Jeannie Lee are exploring ways to potentially reactivate silenced genes, providing new therapeutic avenues for affected individuals.
What are the implications of chromosomal research on X chromosome inactivation for future therapies?
Chromosomal research on X chromosome inactivation reveals mechanisms that may be harnessed for future therapies targeting genetic disorders linked to the X chromosome. Understanding how to manipulate this process could lead to innovative gene therapies that selectively restore the function of healthy genes while minimizing effects on other genes, offering hope for conditions like Fragile X Syndrome and Rett Syndrome.
How could X chromosome inactivation research influence the treatment landscape for Rett Syndrome?
Research on X chromosome inactivation is pivotal for the treatment landscape of Rett Syndrome. By targeting the Xist gene and its role in chromosomal silencing, scientists are developing strategies to potentially reactivate healthy genes that are currently inactive, paving the way for novel therapeutic approaches that could enhance patient outcomes in this severe neurodevelopmental disorder.
| Key Points |
|---|
| The X chromosome inactivation process in females is crucial for balancing gene dosage between sexes. |
| Jeannie T. Lee’s research has advanced understanding of how X chromosome inactivation occurs. |
| The inactivation is facilitated by a gelatinous substance that organizes chromosomes, compared to Jell-O. |
| Xist RNA molecule plays a key role in altering the properties of the surrounding gelatinous substance. |
| Potential therapies for Fragile X Syndrome and Rett Syndrome could be developed by UNSILENCING X-linked genes. |
| Restoring function of mutated genes via X-chromosome unsilencing shows promise for treatments with minimal side effects. |
| Lee’s lab is working towards clinical trials for new treatments leveraging discoveries about X chromosome inactivation. |
| Continued research is needed to understand why other X chromosome genes remain unaffected during these processes. |
Summary
X chromosome inactivation is a complex biological process essential for females, who possess two X chromosomes, to avoid gene dosage imbalances. Recent research led by Jeannie T. Lee at Harvard Medical School has shed light on how cells orchestrate this inactivation, especially through the action of the Xist RNA molecule and surrounding gelatinous materials that facilitate this genomic silencing. The implications of this research are far-reaching, as they open pathways for potential treatments of genetic disorders like Fragile X and Rett syndrome by targeting and restoring function to previously silenced genes. As the scientific community continues to explore these mechanisms, the hope is to translate this knowledge into effective therapies that can alleviate the impact of X-linked genetic diseases.