X-Chromosome Inactivation: Breakthrough for Genetic Disorders
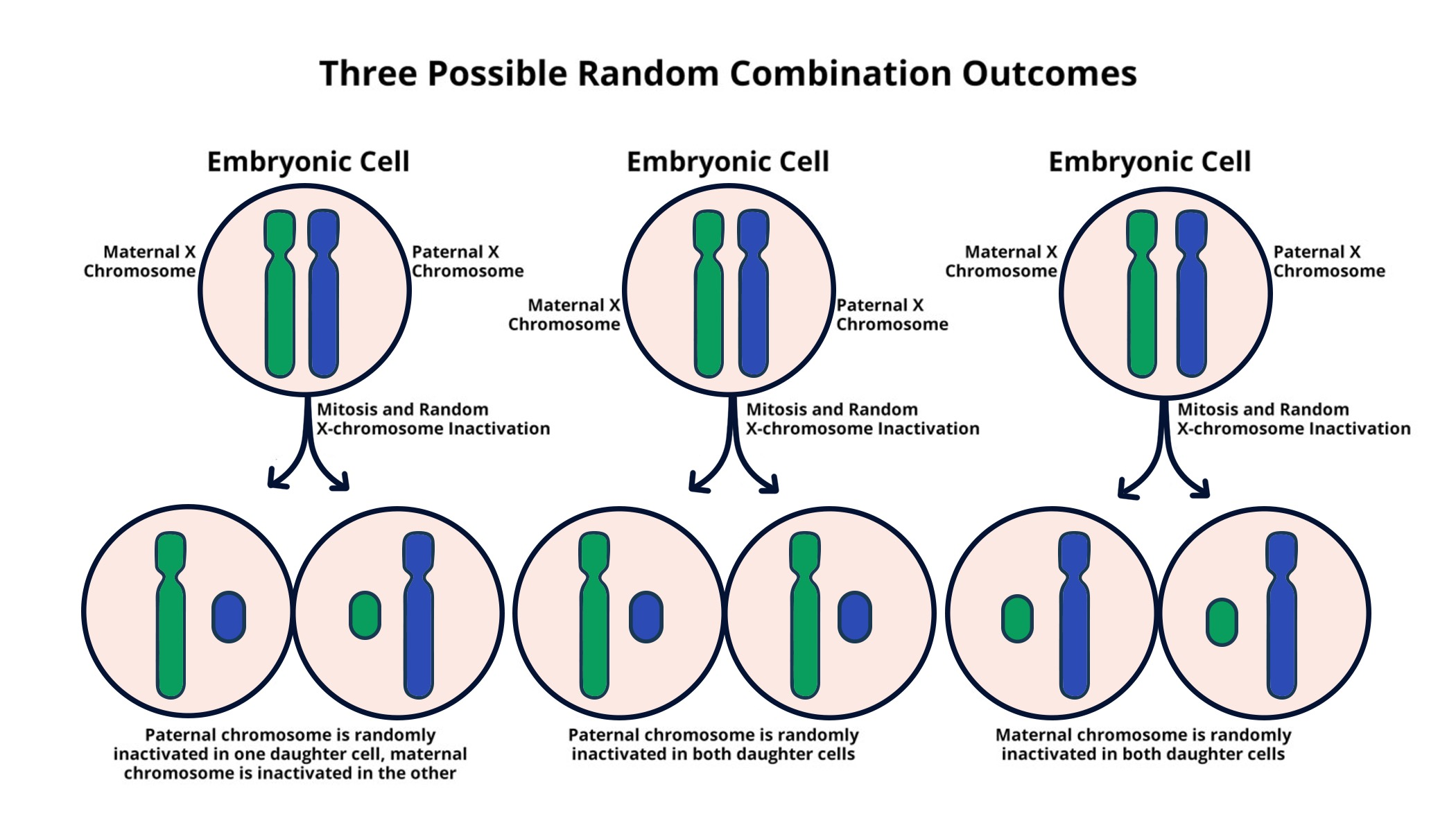
X-chromosome inactivation is a remarkable biological process that balances gene expression in females who possess two X chromosomes. This phenomenon is crucial in understanding genetic disorders like Fragile X Syndrome and Rett Syndrome, which are associated with mutations on the X chromosome. Researchers, including Jeannie T. Lee and her lab at Harvard Medical School, have discovered how the Xist RNA molecule plays a pivotal role in chromosomal silencing, effectively rendering one of the X chromosomes inactive. As they delve deeper, the findings reveal potential pathways for developing treatments that could mitigate the effects of these debilitating diseases. The intricate dance between Xist and the cells’ gelatinous environment sets the stage for groundbreaking advancements in genetic disorders treatment, leading to new hope for those affected.
The inactivation of one X chromosome in females, often referred to as random X-chromosome silencing, is a fascinating mechanism critical to the understanding of sex-linked genetic conditions. This process ensures that gene dosage remains equivalent between males and females, thus preventing potential overexpression of X-linked genes. Notably, conditions such as Fragile X Syndrome and Rett Syndrome shed light on the importance of this silencing mechanism, as they are caused by mutations on the X chromosome. By focusing on the Xist RNA molecule, scientists are unlocking the doors to innovative treatments that could address these and other related chromosomal disorders. Understanding how this chromosomal orchestration works not only clarifies the genetic landscape but also guides future research aimed at effective interventions.
Understanding X-Chromosome Inactivation
X-chromosome inactivation (XCI) plays a crucial role in ensuring that females, who possess two X chromosomes, do not express double the amount of X-linked genes compared to males. This process involves the silencing of one X chromosome in every female cell, which allows for equal gene dosage across sexes. Recent studies focus on the molecular mechanisms underlying XCI, particularly the role of the Xist RNA molecule, which is essential for initiating this chromosomal silencing. By coating the X chromosome, Xist alters the biophysical properties of the surrounding chromatin, effectively making it inactive.
The complexity of X-chromosome inactivation extends beyond mere silencing; it involves a dynamic interaction within the cellular environment. The gel-like substance surrounding chromosomes aids this process by creating secure compartments that prevent chromosome tangling. Researchers like Jeannie Lee have explored how Xist interacts with this Jell-O-like substance, unraveling how it changes from a stiffer to a more liquid state, allowing access for other molecules that aid in the inactivation. This insight into XCI not only broadens our understanding of basic biology but also has promising implications for the treatment of genetic disorders linked to the X chromosome.
Potential Treatments for Fragile X Syndrome and Rett Syndrome
The link between X-chromosome inactivation and genetic disorders such as Fragile X Syndrome and Rett Syndrome is becoming increasingly clear. Mutations leading to these conditions often reside on one of the X chromosomes. As researchers delve deeper into the dynamics of XCI, they are uncovering innovative treatment possibilities that leverage the ability to unsilence inactivated X-linked genes. Jeannie Lee’s lab is at the forefront of this research, with approaches aimed at developing compounds that could reactivate the healthy copies of genes associated with these disorders.
The potential of these therapies is significant, especially considering that many mutations exist on one X chromosome, while the other may harbor a normal version of the gene. By freeing these inactivated chromosomes, currently available only in isolated cell models, there lies hope for reversing the effects of Fragile X and Rett syndromes. The lab’s advancements suggest that these strategies could safely restore gene function while minimizing impacts on healthy genes—an encouraging prospect for patients and their families.
As Lee notes, moving these findings into clinical trials could mark a significant step forward in the treatment of X-linked disorders. With further optimization and safety testing underway, the acceleration towards making these treatments a reality could provide relief to those affected by these debilitating conditions.
The Role of Xist RNA in Chromosomal Silencing
The Xist RNA molecule has emerged as a pivotal player in the process of X-chromosome inactivation. When the X chromosome needs to be silenced, Xist is produced from the chromosome itself and spreads along its length, coating it completely. This ceaseless interaction between Xist and the surrounding chromatin alters the structural features of the ‘Jell-O’ substance that envelops the chromosome, facilitating tighter packing and ultimately leading to gene silencing. This process has major implications not just for understanding normal biology but also in addressing various genetic disorders.
Furthermore, research indicates that the functions of Xist extend beyond merely signaling the inactivation process. The way in which Xist interacts with other molecules strengthens our understanding of chromosomal behavior and its implications for gene expression. As scientists like Jeannie Lee identify the mechanisms by which Xist executes its silencing role, they also uncover the mechanisms that could be targeted for therapeutic interventions—an approach that could ultimately harness the body’s own systems to treat X-linked conditions such as Fragile X Syndrome and Rett Syndrome.
Chromosomal Dynamics and Disorders
The dynamics of chromosomal silencing are not only fascinating but also essential in the context of genetic disorders. The delicate balance of gene expression orchestrated by the inactivation of one X chromosome ensures that females maintain gene dosage parity with males. Yet, mutations on the X chromosome can disrupt this balance, leading to conditions such as Fragile X and Rett syndromes. Understanding how this silencing occurs offers insights into potential avenues for treatment.
The exploration of chromosomal dynamics highlights opportunities for novel therapeutic strategies. By leveraging advances in genomics and molecular biology, researchers are not only beginning to decode the complexity of X-linked disorders but are also developing targeted treatments that can reactivate silenced genes. This ongoing research underscores a paradigm shift in the approach to genetic disorders, emphasizing the importance of a deep understanding of cellular mechanisms for effective treatment development.
Clinical Implications of X-Chromosome Research
The clinical implications of research into X-chromosome inactivation are profound as they hold the promise of therapeutic breakthroughs for genetic disorders. With teams like Jeannie Lee’s investigating the potential to unsilence X-linked genes, the path toward clinical applications becomes clearer. Existing health disparities among individuals with X-linked disorders underscore the urgent need for effective treatments that can mitigate their impacts.
Future clinical trials resulting from these pioneering studies will determine the safety and efficacy of emerging therapies based on X-chromosome dynamics. This phase marks a crucial step not only in validating laboratory findings in live patients but also in demonstrating the real-world applicability of this research. The anticipation of transitioning from basic science to clinical application offers hope to those affected by conditions such as Fragile X Syndrome and Rett Syndrome, providing pathways for improved health outcomes.
Insider’s View on Genetic Disorders Treatment
Insiders in the field of genetics continually emphasize the transformative potential of understanding chromosomal biology for developing therapeutic strategies. Within this scope, the insights on X-chromosome inactivation serve not just academic purposes but are rapidly evolving into practical applications. With gene-targeted therapies on the horizon, the treatment landscape for genetic disorders is poised for a significant shift.
Jeannie Lee’s work illustrates the crucial role that foundational research plays in paving the way for real-world health solutions. With continued support from institutions such as the National Institutes of Health, breakthroughs in understanding chromosomal dynamics can translate into clinical applications. As research progresses, the commitment to addressing the unmet medical needs of individuals with Fragile X Syndrome and Rett Syndrome stands as the primary objective.
Exploring Genetic Mechanisms Behind X-Linked Conditions
The exploration of genetic mechanisms behind X-linked conditions remains a significant area of inquiry, especially as researchers aim to decipher the interplay between X-chromosome inactivation and gene expression. Understanding how mutations influence the silencing process not only shines a light on disease mechanisms but may also guide future therapeutic approaches. Researchers like Jeannie Lee have shed light on the role of the Xist RNA molecule amid this complex phenomenon, highlighting critical interactions essential for therapeutic advancement.
The ongoing research efforts focus on unveiling the genetic undercurrents that lead to conditions like Fragile X and Rett Syndromes. The knowledge garnered from this research enhances our understanding of how effective treatments can be developed. This proactive approach ensures that emerging therapies are built on a solid foundation of scientific knowledge that addresses the root causes of the disorders, potentially transforming the prognosis for affected individuals.
Moving Toward Clinical Trial Readiness
As cutting-edge research transitions from laboratory settings to clinical trial readiness, the focus increasingly shifts to ensuring safety and efficacy of new treatments derived from findings on X-chromosome inactivation. The goal is to refine methodologies and experimental designs that translate into successful clinical interventions. By validating preclinical results in appropriate trial contexts, researchers are setting the stage for meaningful advancements in the treatment of genetic disorders.
The journey from discovery to clinical application is complex but essential for making effective therapies available to patients. As scientists optimize experimental approaches and navigate regulatory pathways, the aspiration remains clear: to provide hope for individuals living with inherited disorders such as Fragile X and Rett Syndrome. This pivotal phase of clinical research holds the promise of not only improving individual health outcomes but also reshaping the landscape of genetic disorder treatment.
The Journey of Understanding Chromosomal Silencing
The investigative journey into understanding chromosomal silencing, especially concerning X-chromosome inactivation, represents decades of dedicated scientific inquiry. Each discovery builds upon previous knowledge, illustrating the complexity of cellular processes at play, particularly in females with two X chromosomes. This extensive body of work reflects an evolving understanding of how genetic disorders can arise and be treated.
Moreover, the overarching commitment of researchers emphasizes that foundational scientific inquiry is the cornerstone for therapeutic discoveries. As exemplified by Jeannie Lee’s lab, the exploration of the X chromosome will continue to yield insights that could transform the lives of countless individuals globally, ushering in new treatment modalities for some of the most challenging genetic disorders known today.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important in genetic disorders like Fragile X Syndrome and Rett Syndrome?
X-chromosome inactivation is a biological process where one of the two copies of the X chromosome in female mammals is silenced to prevent an overexpression of genes. This is crucial for maintaining gene dosage balance and can directly relate to genetic disorders such as Fragile X Syndrome and Rett Syndrome, both of which are linked to mutations on the X chromosome. Understanding this process can aid in developing potential treatments for these conditions.
How does the Xist RNA molecule contribute to X-chromosome inactivation?
The Xist RNA molecule plays a central role in X-chromosome inactivation by coating the X chromosome and changing the properties of the chromosomal environment, often described as gelatinous ‘Jell-O’. This alteration allows for the silencing of the X chromosome, thus preventing the expression of potentially harmful genes, which is especially relevant in genetic disorders caused by X-linked mutations.
What is the significance of chromosomal silencing in relation to potential treatments for genetic disorders?
Chromosomal silencing, specifically X-chromosome inactivation, is significant because it can be targeted to ‘unsilence’ inactivated genes on the X chromosome that carry mutations. This is particularly promising in the context of treating genetic disorders like Fragile X Syndrome and Rett Syndrome, where restoring function to these genes could alleviate symptoms and enhance quality of life.
Are there new therapeutic approaches being explored for Fragile X Syndrome and Rett Syndrome related to X-chromosome inactivation?
Yes, researchers are exploring therapeutic approaches to optimize X-chromosome inactivation techniques in order to unsilence mutated genes. Advances in research led by Jeannie Lee’s lab suggest potential treatments that may correct the effects of genetic disorders like Fragile X Syndrome and Rett Syndrome, thus opening the door for future clinical trials.
What challenges remain in understanding X-chromosome inactivation and its application in genetic disorder treatment?
Despite progress in understanding X-chromosome inactivation, challenges remain, such as why the restoration of function appears to primarily affect mutated genes rather than healthy ones. Research is ongoing to fully unravel the mechanisms behind X-chromosome inactivation and to ensure that any derived therapies can target genetic disorders effectively while minimizing side effects.
| Key Point | Details |
|---|---|
| X-Chromosome Challenge | Females have two X chromosomes, while males only have one, requiring females to inactivate one of their X chromosomes. |
| Role of Jeannie Lee’s Lab | Jeannie Lee’s lab at Mass General has focused on understanding X-chromosome inactivation. |
| Discovery of Xist | A gene on the X chromosome produces an RNA molecule called Xist, which plays a crucial role in inactivating it. |
| Mechanism of Inactivation | Xist interacts with a gelatinous substance surrounding chromosomes, changing its properties and facilitating inactivation. |
| Potential Treatments | Freeing inactivated X chromosomes could potentially treat certain genetic disorders, such as Fragile X and Rett syndromes. |
| Clinical Research Progress | Ongoing optimization of methods to unsilence X-linked genes, with plans for clinical trials. |
| Future Implications | Restoring mutated gene function while minimizing effects on healthy genes could lead to effective treatments. |
| Research Support | The research was funded by the National Institutes of Health. |
Summary
X-chromosome inactivation is a vital biological process that enables females to balance gene expression between their two X chromosomes. This groundbreaking research led by Jeannie Lee’s lab unveils the intricate mechanisms behind this process, highlighting the role of the Xist RNA molecule and the surrounding gelatinous substance that facilitates inactivation. The implications for treating genetic disorders such as Fragile X and Rett syndromes are profound, as freeing inactivated X chromosomes may allow for the utilization of healthy gene versions and open new avenues for clinical applications.